Differentiation is more than a word

On a regular basis we see and hear comments about cost saving measures for pharmaceuticals. Too often is the plasma protein sector immediately drawn into this debate because many persons do not understand why this sector is so different from what we call traditional pharmaceuticals.
Plasma protein therapies (PPT’s) are used by persons with rare diseases. Together they represent small patient populations. We are not talking about blood pressure medication, diabetes therapies or psychiatric drugs used by millions of people. That is already a differentiator. It is important to understand that the costs for the development and manufacturing of plasma protein therapies are born by a relatively small group of individuals, Because of that we end up with a relatively higher price per unit.
Some recipients of PPT’s e.g. persons with hemophilia have a choice between therapies. These include plasma derived clotting factors, recombinant clotting factors, monoclonal therapies and even gene therapy. Other recipients of PPT’s do not have a choice and depend completely on the supply with plasma derived medicinal products. The manufacturing of these therapies are very different than the products that are made from chemical compounds.
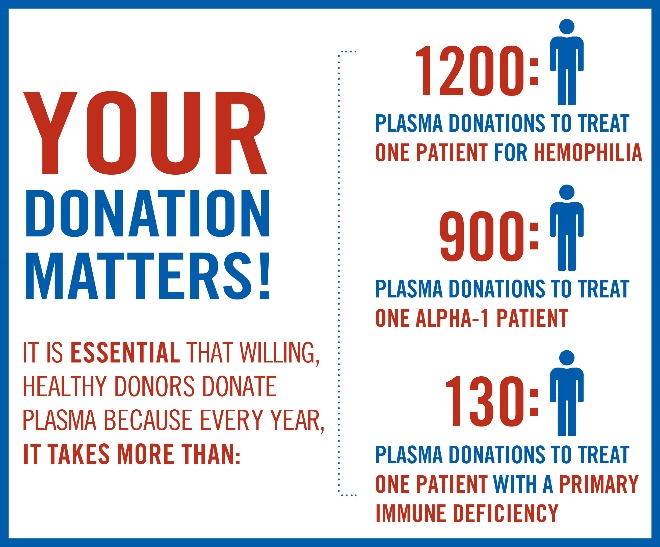
The starting material is human plasma from committed healthy donors who are willing to donate on a regular basis to help their fellow citizens. The number of donations needed for a year of therapy are enormous: 130 for immunodeficiency, 900 for alpha-1-anti-trypsine deficiency and 1200 for one year of treatment of Factor VIII. It is not difficult to understand that the costs of these many donations and plasma are substantial higher than the production of a chemical compound.
That is not all. It is just the beginning. There is a strict donor selection procedure that includes medical checks and diagnostic checks to ensure that the donor is not carrying an infectious agent. After the donation, the plasma is kept in Inventory Hold for at least 60 days before the manufacturing can start. This gives an additional level of safety because it allows to obtain post-donation information. If that information would have been known at the time of the donation and a reason for disqualification of the donor, this additional step ensures that this donation can be traced and discarded before the manufacturing process starts. Having the inventory just sitting there for 60 days is an enormous financial investment and capital block that does not exist for other industries.
Then the manufacturing process starts and involves separation, purification and inactivation just to name a few. Every step involves robust Quality Control and Quality Assurance measures. It does not stop there. After the distribution to a patient there is monitoring and vigilance.
Unlike the manufacturing of traditional pharmaceuticals, the time between donation and distribution to the individual that needs therapy, is between 7 and months!
It is good to see that more and more persons are starting to see why the plasma protein industry is unique and cannot be subjected to the same cost containment measures as we see for pharmaceutical drugs. But it is not enough. We need to continue to help more people understand the different nature of the plasma protein industry.
Jan M Bult